lv purification|lentivirus purification process : 2024-10-06 A scalable and GMP compatible purification process for LV is proposed and several improvements were described for the different purification steps. Firstly, distinct . The 10 Most Affordable Audemars Piguet Watches in 2024. Designed to serve as a beacon for both new enthusiasts embarking on their horological journey and .
0 · lentivirus purification process
1 · lentivirus purification
2 · Altro
Check out our collection of pre-loved Audemars Piguet Jules Audemars. Remember, our pre loved Jules Audemars watches come with a 24 month warranty. +44 1622621392
lv purification*******LVs produced in most research laboratories contain contaminants that can generate confounding effects in experimental studies. Soldi et al. describe a laboratory-scale workflow for purified LV .
Advances in Lentivirus Purification. Abstract. Lentiviral vectors (LVs) have been increasingly used as a tool for gene and cell therapies since they can stably integrate . A scalable and GMP compatible purification process for LV is proposed and several improvements were described for the different purification steps. Firstly, distinct . Ruscic et al. reported LV purification using regenerated cellulose nanofibers derivatized with a quaternary amine and produced by electrospinning. The authors claimed to achieve a volumetric . Lentiviral vectors (LVs) are used in cell and gene therapies due to their ability to transduce both dividing and non-dividing cells while carrying a relatively large genetic . Nanofibers have been used to successfully purify lentiviral vectors (Ruscic et al. 2019) and adenoviral vectors (Turnbull et al. 2019) by anion-exchange .
Purification by sucrose gradient ultracentrifugation abolished the immune response, but vector titers also decreased substantially. Lentiviral vector production in the absence of serum in.Detailed herein is a simple protocol for the production LV vectors, describing the triple transfection of an LV transfer vector and LV helper plasmids into HEK-293 cells, and the .
Lentiviral vectors (LVs) have gained value over recent years as gene carriers in gene therapy. These viral vectors are safer than what was previously being used for .
Purification is a service offered by Olra in exchange for Rift Crystals to cleanse cursed, unusable artifacts to obtain helpful equipment and items. Cursed objects can only be found on Bitterblack Isle. Cursed items on .Given the significant contribution that LV vectors make to the overall cost of goods of these cell therapy products, and the importance of maximizing quality for patient safety and regulatory CMC purposes, it is imperative .Gibco LV-MAX Lentiviral Production system uses our GMP-manufactured transfection reagent, supplement, HEK 293-derived cells, . purification etc.). Can I transfect before passage 5 (P5)? We recommend that cells . The purification train involves AEX capture with a DEAE-650C resin, concentration with TFF via 100 kDa hollow fiber or cassette (both performed comparably, and polishing and buffer exchange with gel filtration with Sepharose 6FF resin. Ghosh et al. (2022) investigated and optimized processes for LV purification using four IEX resins . High-titer LV stocks were achieved in 293T cells grown in suspension. Produced viruses were accurately characterized and the functionality was also tested . Analytical assays after LV purification.Gibco LV-MAX Lentiviral Production system is designed for facilities aiming at therapeutics by offering our transfection reagent, supplement, HEK 293-derived cells, and media enhancer. . For downstream purification of virus, .Lentiviral (LV) vectors offer unique advantages over other gene delivery systems, namely the ability to integrate transgenes into the genome of both dividing and nondividing cells. Detailed herein is a simple protocol for the production LV vectors, describing the triple transfection of an LV transfe .
Different purification methods are utilized throughout the process. . (LV), shown in Figure 1. The variety of fibrous protein spikes, lipid bilayers, spike proteins, and genomic identity create a large matrix of structures that can complicate the downstream processing of viral vectors.
Production of adeno-associated virus vectors for in vitro .
Ghosh et al. (2022) investigated and optimized processes for LV purification using four IEX resins. 147 Capto Q ImpRes provided impressive infectious recoveries of ~100% and an HCP LRV clearance .
Lentiviral vectors (LVs) have recently witnessed an increasing demand in research and clinical applications. Their current purification processes represent the main bottleneck in their widespread use, as the .lv purification lentivirus purification process This is not adequate for LV purification, since binding sites located within particle pores are typically not accessible to the considerably larger viral vectors; therefore, alternative stationary phases are necessary. 7 Cellulose nanofibers are a new scalable purification platform. In addition, strategies, such as affinity-based isolation of vector particles, could further increase LV purification yield, as recently described for the capture of cTag8-expressing viral particles. 49 Finally, this platform could be further developed toward a more versatile workflow, compatible also with other transfection protocols (e.g . Heparin has been used for LV purification, recovering up to 53% of viral vectors and removing up to 94% of protein impurities and 56% of residual DNA [78,84]. NaCl is routinely used to elute vectors from heparin columns as the binding of vectors through their surface molecules is all based on charge–charge interactions of positively . DOI: 10.1002/btpr.3286 Corpus ID: 250388194; Evaluation of lentiviral vector stability and development of ion exchange purification processes @article{Ghosh2022EvaluationOL, title={Evaluation of lentiviral vector stability and development of ion exchange purification processes}, author={Ronit Ghosh and .
An optimized method for high-titer lentivirus preparations . This is because you have a weekly Purification limit in Once Human. As a result, using lower level Cortex is terribly inefficient. The best way to maximize Starchrom is by having you and your hive grinding to get Cortex Lv 3. This is because they give 100 Starchrom each. On the other hand, Lv 2 gives 85 Starchrom each and Lv 1 gives 60 . The purification of LV is examined, evaluating different sequences of downstream process operations for both small- and large-scale production requirements. For scalable operations, a key focus is the development in chromatographic purification in addition to an in-depth examination of the application of tangential flow filtration. Large-scale purification of functional AAV particles .CP Calculator - Pokémon GO - GameInfo
lv purificationPurification LV is on Facebook. Join Facebook to connect with Purification LV and others you may know. Facebook gives people the power to share and makes the world more open and connected.lentivirus purification process M.S. optimized and performed the experiments (downstream LV purification and quality controls) with help of L.S.S. (upstream LV production), analyzed the data, and wrote the manuscript. G.U. performed gene editing experiments. T.K. and A.A. generated and analyzed the data on IFN-α LV and cytokine concentration in plasma.
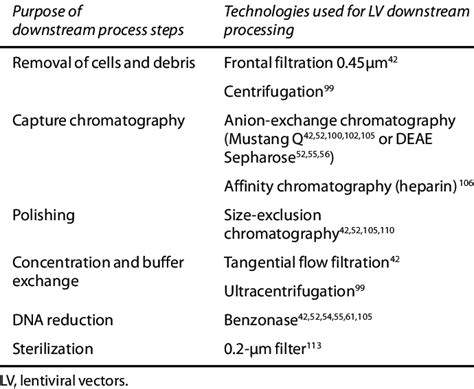
Download Table | Standard downstream process technologies applica- ble to LV purification and recovery from publication: Production of lentiviral vectors | | ResearchGate, the professional network .

1. Introduction. Lentiviral vectors (LV) represent one of the three most commonly used viral vectors for gene transfer in gene therapy clinical trials [1] and are the most frequently used viral gene delivery platform for the ex vivo generation of chimeric antigen receptor (CAR)-T cells for cancer immunotherapies [2].The LV size offers a high . Functional recoveries are essential to be able to value the success of the purification steps; however, p24 ELISA-based total viral particle (vp) recovery remains a sufficient method for a quick and noncell-based check for the overall LV recovery of a process step. 18 A comparison between vp concentration and transducing units (TUs) .Large-scale lentiviral vector (LV) concentration can be inefficient and time consuming, often involving multiple rounds of filtration and centrifugation. This report describes a simpler method using two tangential flow filtration (TFF) steps to concentrate liter-scale volumes of LV supernatant, achi .
In this Levoit Core 200S vs LV-H132 review, we will compare two of the entry-level models of the Core and LV series from the fast-growing American brand for small home appliances. Both were created as compact and budget-friendly air purifiers for small rooms. The LV-H132 was one of Levoit’s best-selling devices, but is no longer .When Nahida hits an opponent affected by All Schemes to Know 's Seeds of Skandha with Normal or Charged Attacks after unleashing Illusory Heart, she will use Tri-Karma Purification: Karmic Oblivion on this opponent and all connected opponents, dealing Dendro DMG based on 200% of Nahida's ATK and 400% of her Elemental Mastery. .
Online op zoek naar herenschoenen van adidas? Zoek niet verder en shop nu alle modellen, stijlen en kleuren mannenschoenen in onze officiële shop.
lv purification|lentivirus purification process